Big picture: human embryonic stem cells as a model for the embryo
How an amorphous lump of identical cells is able to develop into a human being is a profound question of equal intellectual and practical importance. Its fascination springs in part from the desire to know where we come from, and how we differ from other animals. Yet the ability to direct differentiation of cultured cells into distinct cell types for therapeutic use, perhaps even coax them to form complete organs, relies on answering the same question. Ethical considerations make experimentation on human embryos unacceptable and on other mammals undesirable. Moreover, significant interspecies differences complicate inference about human development from animal models.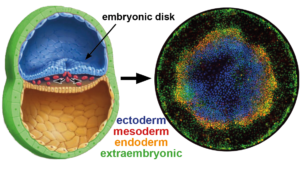 This challenges us to find answers in alternative ways.
This challenges us to find answers in alternative ways.
Remarkably, human embryonic stem cells (hESC) retain the embryo’s innate capacity to self-organize. The embryonic body plan is laid out during gastrulation, when pluripotent cells commit to one of three different lineages -ectoderm, mesoderm or endoderm- that organize into three germ layers. Upon treatment with the differentiation-inducing molecule BMP4, hESCs confined to a disc by micropatterned substrate mimic this process and reproducibly differentiate into concentric rings corresponding to each germ layer. Importantly, the same genetic circuitry as in the embryo is orchestrating this pattern. Therefore, by systematically varying the environment we can probe underlying mechanisms with throughput and precision unachievable in living embryos.
Read more about stem cells as a model for embryonic patterning, or as a platform to study early human development, in one of our recent reviews.
Research subjects
Live imaging of signaling in hESCs: fluorescent signal transducer moves into nucleus in response to morphogen
Morphogen dynamics
Morphogens are diffusible signaling molecules thought to determine cell fates in a concentration-dependent way, but this static model is insufficient for the vertebrate embryo, where differentiating cells experience rapidly changing morphogen levels. On theoretical grounds morphogen dynamics can have a dramatic effect on the way pattern formation takes place. Nodal and BMP4 are crucial signaling factors shaping the mammalian embryo, without which gastrulation is completely disrupted. To address how cells interpret changing morphogen levels, we characterized signaling, transcription, and differentiation of hESCs in response to BMP4 and Nodal while modulating the rate of concentration change. Breaking with the current paradigm, we discovered that response of many Nodal target genes depends on the rate of concentration increase, in contrast to BMP4 response, which is concentration dependent. Moreover, we found that micropatterned colonies generate a wave of endogenous Nodal signaling, so that cells experience rapidly increasing Nodal specifically where mesoderm and endoderm form. Our goal now is to understand how these rapidly changing signaling gradients are generated and translated into spatial domains of gene expression during self-organized patterning. An important part of that is to understand how the interpretation of signals changes as the cells differentiate. Read more about interpretation of morphogen dynamics in our recent paper.
Mechanical forces in pattern formation
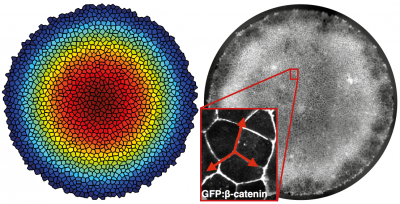
In recent years there has been a surge of work on cellular and tissue mechanics, and an important role for mechanics in growth regulation and differentiation has been demonstrated in a number of systems. Little is known about the role of mechanics in gastrulation or self-organization of stem cells, but there is good reason to believe that it is significant. The primitive streak is the embryonic region where mesoderm and endoderm form and ingress, and it is likely to have distinct mechanical properties from the rest of the embryonic disc due to the dissolution of the basement membrane and migration of cells toward the streak. By combining a stem cell model for gastrulation with force inference methods and mechanical manipulation of substrate and intracellular forces, we can understand the effect of mechanics on germ layer specification and spatial organization of hESCs.
Role of tissue geometry and topology in patterning
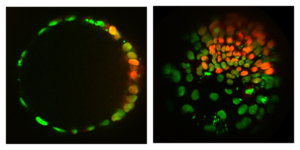
Understanding the interplay between pattern and shape, through which the body plan is established, is a major challenge in developmental biology. Unlike in embryos, geometry (shape) and topology (how things are connected) of stem cell colonies can be controlled to study the effect on patterning. The simplest models for spatial differentiation in micropatterned stem cell colonies depend on the existence of a colony border. However, by growing cells on spheres we found that patterning still occurs. This suggests that the underlying mechanism is the spontaneous formation of a morphogen gradient through an activator-inhibitor system, as suggested long ago by Alan Turing. Visualizing endogenous morphogens will conclusively show this. Looking at patterning on surfaces with different symmetry and shape will further constrain the mechanism of symmetry breaking and provide insight into the establishment of the body axes. Moreover, developing methods to control the 3D geometry of stem cell colonies will help reduce variability in organoid formation, which will enable the quantitative comparison between individual organoids that is needed for systematic study and fulfillment of their potential as a disease model.